ThinPrep® PreservCyt® Collection Vials
The cervical sample collection and preservation that is tested and trusted by healthcare professionals all over the world.
Information for Healthcare Professionals

Overview
Documents
A Wealth of Knowledge in a Single Vial
The ThinPrep® Pap Test collection vial is used globally for cervical sample collection and preservation, trusted by healthcare professionals around the world. More than 1 billion ThinPrep vials have been used globally for cervical cancer screening. Only a single sample is required for both cytology and molecular testing.1 In addition to the Aptima® HPV assay, Aptima Combo 2® and Aptima® Trichomonas vaginalis Assays can also be tested from the vial.
Trust the Track Record
The ThinPrep Pap Test collection vial enables an optimal preservation of cellular material, mRNA and DNA.
Visualisation
Significantly more effective than conventional Pap smear for the detection of Low-grade Squamous Intraepithelial Lesion (LSIL) and more severe lesions (HSIL).2
Detection
Improved ability to detect glandular disease compared to conventional Pap.2
Proven
Approved for use with all leading FDA-approved, CE-marked and ARTG included HPV tests.

Working With You to Advance Cervical Health
We pride ourselves on aiming to be champions of women’s health and global leaders in screening, dedicated to advancing the accuracy and early detection of cervical cancer. From HPV to cytology, and now also AI-based digital diagnostics, we offer a comprehensive screening portfolio, from sample collection to diagnosis.
Advancing the Early Detection of Cervical Cancer
30+ years
of longitudinal data supporting ThinPrep performance3
1+ Billion
ThinPrep Pap tests have been performed4
59.7% Higher HSIL
detection than conventional pap testing2
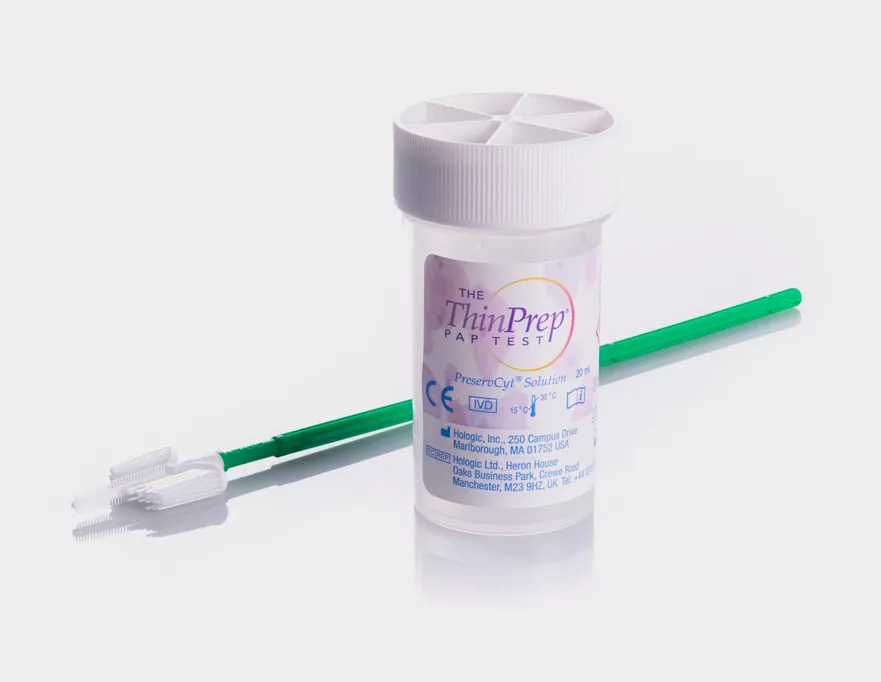
The ThinPrep Pap Test Product Design
Trusted performance across all grades of cervical disease:
- Safety seal, torque mark and fill level mark
- Collection device rinsed in ThinPrep Vial.2
- Significantly more epithelial cells collected compared to conventional pap.5
- Immediate fixation maintains cell quality.5
- 6 weeks storage at room temperature for cytology.2
- Methanol base (no formaldehyde) protects molecular content for analysis.6
- 4mL pre-aliquot removal volume facilitates multiple ancillary tests.2
ThinPrep Specimen Collection with Non-Gyn Application
These solutions have a wider application beyond cervical health,and come with a standardised workflow. Browse options for collection and cytology preparation, including fine needle aspirates, urine, effusions, respiratory and gastrointestinal tract sample types.
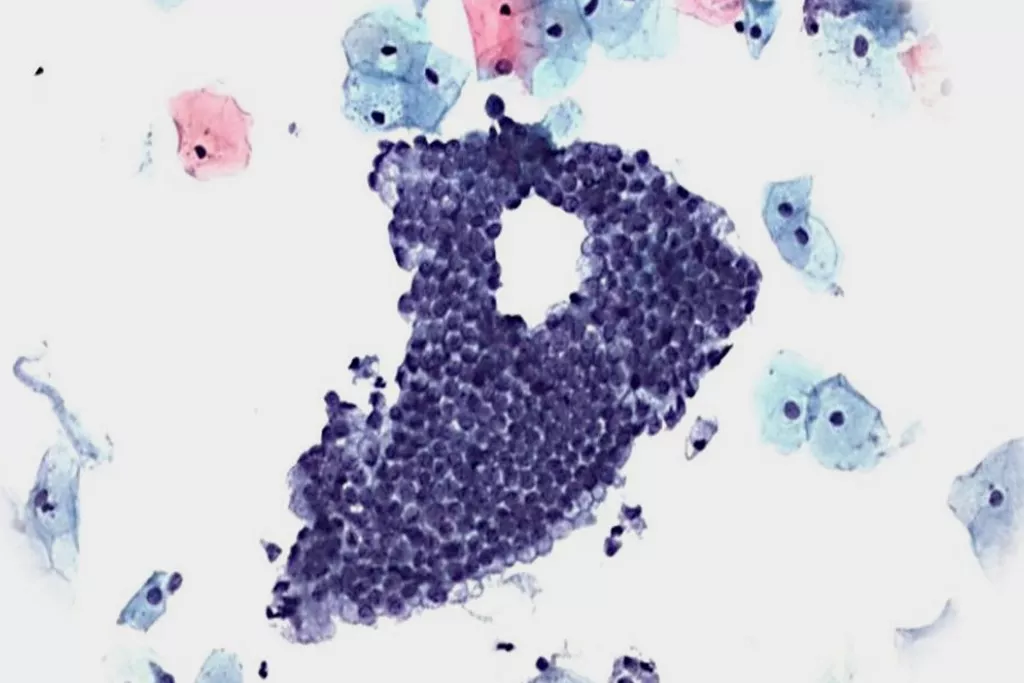
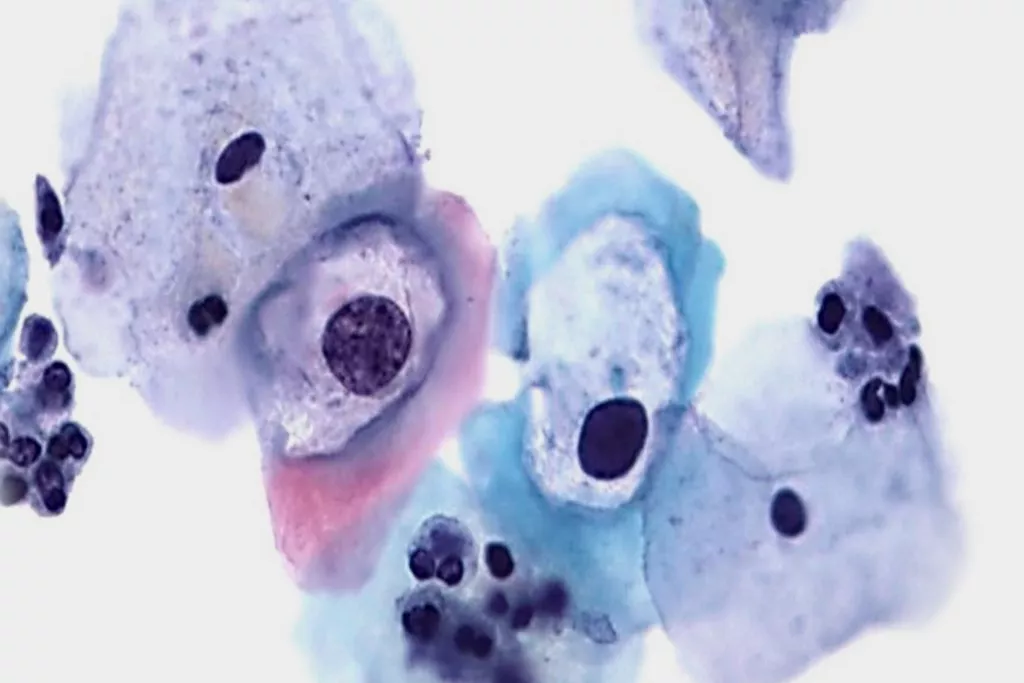
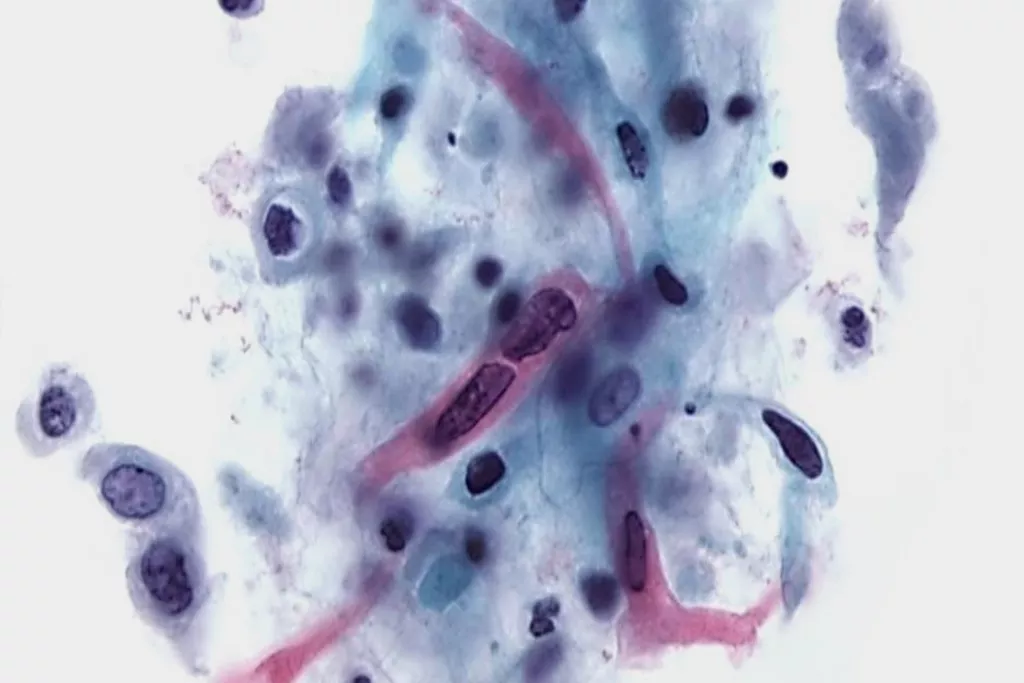
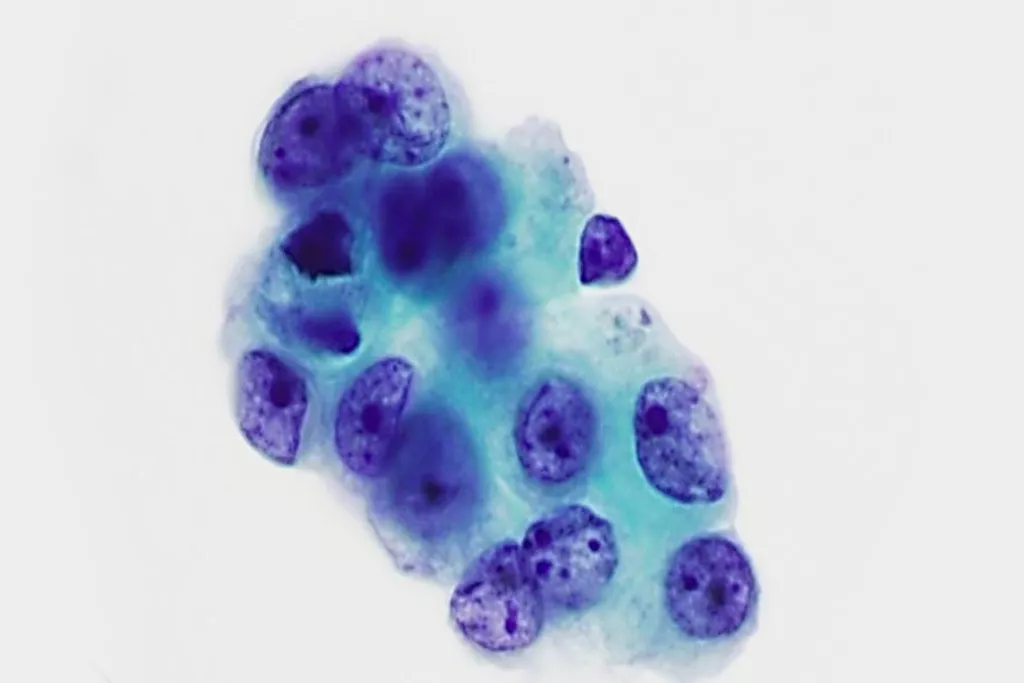
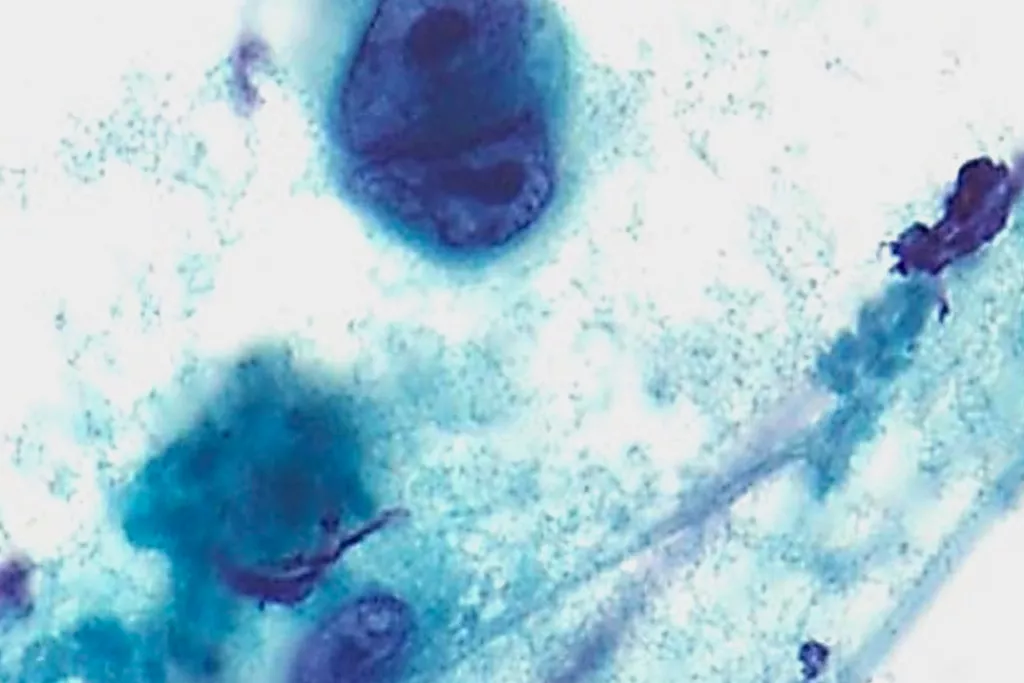
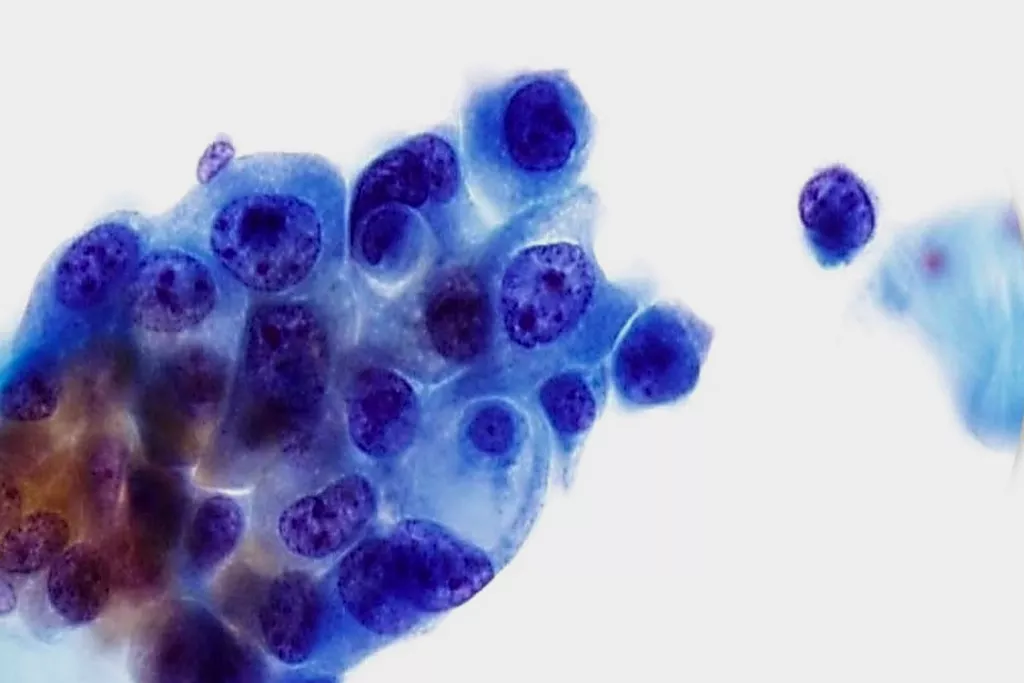
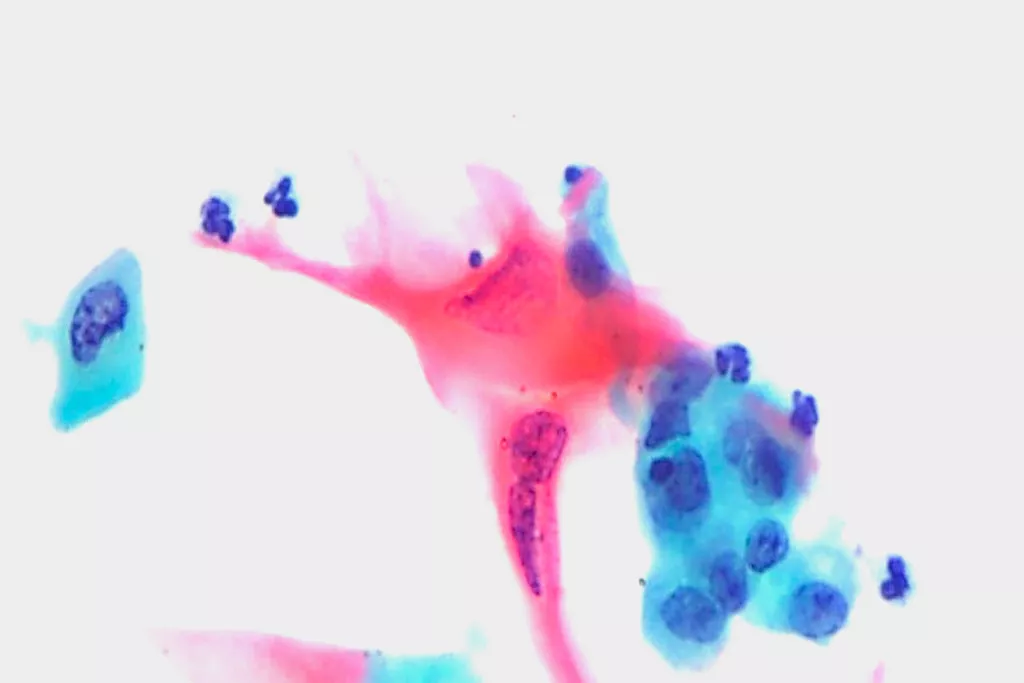
Image Gallery9
A Patient Friendly Experience
Out-of-the-vial testing minimises the number of samples required for multiple test results. For cervical cancer screening in the case of a first positive or abnormal result, the reflex testing can be directly performed from the same primary sample. This versatile application improves patient comfort and avoids additional follow-up.

Insights
* Aptima HPV assay, Aptima HPV 16 18/45 Genotype assay, Cervista HPV HR test, Cervista HPV 16/18 test, Roche cobas HPV test and Hybrid Capture 2 HPV DNA test
ThinPrep® Pap Test PreservCyt Solution, Instructions for Use AW-22719-001 Rev 001
ThinPrep 5000 Processor User Manual, MAN-07493-002 Rev 003
FDA approved since 1996 with hundreds of peer reviewed publications globally
Data based on Hologic sales numbers since launch in 2012 to 31Jan2020. File reference: AHPVtotalnumberJan2020 (Page 6 https://hologic.box.com/s/w5ccn2nza52n5010pr42ivy4ebfqbgxb)
Hutchinson ML, Isenstein LM, Goodman A, Hurley AA, Douglass KL, Mui KK, et al. Homogeneous Sampling Accounts for the Increased Diagnostic Accuracy Using the ThinPrepTM Processor. Am J Clin Pathol. 1994;101:215-9.
Powell N, Smith K, Fiander A. Recovery of human papillomavirus nucleic acids from liquid-based cytology media. J Virol Methods. 2006 137:58-62.
ThinPrep ThinPrep® PreservCyt® Solution, Instructions for Use AW-22939-001 Rev 001
ThinPrep UroCyte PreservCyt, Instructions for Use AW-22938-001 Rev 001
Images received from Cytopathology Department, Llandough Hospital, Wales
Safety Data Sheets
Package Inserts
Related Products
2797
Hologic BV, Da Vincilaan 5, 1930 Zaventem, Belgium.
Notified Body number wherever applicable
EC Representative Information wherever applicable